
4 The current exploratory analysis of KEYNOTE-522 assessed event-free survival according to RCB category. Prior studies have shown the prognostic value of RCB score and RCB categories in quantifying the extent of residual disease after neoadjuvant chemotherapy.
#Keynote 522 2022 plus#
3 The findings led to approval in the United States and Europe for pembrolizumab plus chemotherapy as neoadjuvant therapy, followed by adjuvant single-agent pembrolizumab, in high-risk patients with early-stage triple-negative disease.

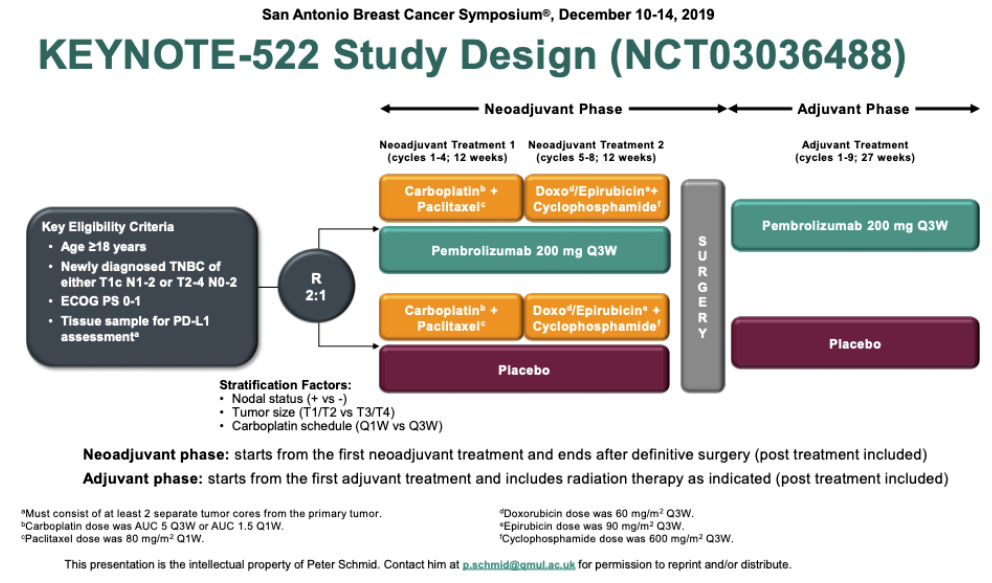
00055) 2 and event-free survival (hazard ratio = 0.63 P =. The primary results showed statistically significant and clinically meaningful improvements in the pathologic complete response rate (a 13.6% absolute increase P =. After definitive surgery, patients also received pembrolizumab or placebo for nine cycles or until recurrence or unacceptable toxicity.

KEYNOTE-522 tested the benefit of adding pembrolizumab to chemotherapy in 1,174 patients with early-stage triple-negative breast cancer. “These results indicate that the event-free survival benefit from pembrolizumab extends to patients who do not achieve a pathologic complete response and suggest there is a contribution from the adjuvant pembrolizumab component of the treatment,” Dr. The smallest subset with extensive residual disease (RCB 3) in both arms (5.1% and 6.7%, respectively) had a poor outcome the 3-year event-free survival rates were 26% and 35%, respectively. Treatment with pembrolizumab plus chemotherapy led to 3-year event-free survival rates of almost 95% for those with an RCB score of 0 and 76% and 85% for those with an RCB score of 1 and 2, respectively. “Also, the addition of pembrolizumab resulted in numerically fewer event-free survival events in the RCB-0 (complete pathologic response), RCB-1 (measurable residual disease), and RCB-2 (moderate amount of residual cancer) categories, with the most pronounced benefit seen in the RCB-2 group.” This indicates that the addition of pembrolizumab not only increased the pathologic complete response rate but shifted RCB to lower categories across the entire spectrum of residual disease,” he reported. “There were a lower percentage of patients in each RCB category in the pembrolizumab group than the placebo group.

There were fewer RCB3’s, which is the poorest prognosis and largest amount of residual cancer, and again, there were fewer of them in the pembrolizumab arm, approximately 5% of patients ended up with still this poor prognosis.- Lajos Pusztai, MD, DPhil Tweet this quote There were fewer RCB2 patients in the pembrolizumab arm because many of them moved to the RCB1 category. That's still significant, but we also noticed that there was a consistent shift to lower amounts of residual cancer across the entire spectrum.Ġ:31 | There were fewer patients in the RCB1 group in the pembrolizumab cohort than in the control cohort because many of them moved into the RCB0.
#Keynote 522 2022 trial#
Lajos Pusztai, MD, DPhil, professor of Medicine (Medical Oncology), and co-leader of Genetics, Genomics, and Epigenetics at Yale Cancer Center, discusses recent data from the phase 3 KEYNOTE-522 trial (NCT03036488) of patients with triple-negative breast cancer (TNBC).ĭata from the trial were presented during the 2022 American Society of Clinical Oncology (ASCO) and showed that an increased residual cancer burden (RCB) score was linked with worse event-free survival (EFS) in this patient population.įor patients administered pembrolizumab (Keytruda) and chemotherapy, there was an improvement in EFS compared with patients enrolled in the control arm who showed lower RBC scores.Ġ:08 | What we reported at ASCO was that in the final analysis, including all patients, the pathological cognitive response rate difference decreased from 13.6%-7.2%.


 0 kommentar(er)
0 kommentar(er)
